CART-related Neurotoxicity (NT) Market Size & Share | CART-related Neurotoxicity (NT) Market Research Report, Market Analysis, Market Growth, trends and Forecast
CART-related Neurotoxicity (NT) Market Insights, Epidemiology and Market Forecast till 2030
DelveInsight launched a new report on CART-related Neurotoxicity (NT) – Market Insights, Epidemiology, and Market Forecast-2030.
DelveInsight’s “CART-related Neurotoxicity (NT) – Market Insights, Epidemiology, and Market Forecast-2030” report delivers an in-depth understanding of the CART-related Neurotoxicity (NT) , historical and forecasted epidemiology as well as the CART-related Neurotoxicity (NT) market trends in the United States, EU5 (Germany, Spain, Italy, France, and United Kingdom) and Japan.
Chimeric antigen receptor (CAR)-T-related Neurotoxicity (NT) is a type of toxicity that is related with the CAR T-cell therapy. Another toxicity associated with the CAR T-cell therapy is known as cytokine release syndrome (CRS). After the initial approval of CAR-modified T (CAR-T) cell immunotherapy, it has produced impressive results in clinical trials treating relapsed and/or refractory B-cell malignancies. In this therapy, T-cells obtained from the patient, or sometimes not as often from an allogeneic donor, are modified to express a CAR, which consists of an extracellular tumor-targeting moiety which is fused to one or more intracellular T-cell signaling domains.
CAR-T NT is a well described clinical phenomenon, however, its pathophysiology still remains inadequately understood. Several studies conducted on animal models suggest the centrality of monocytes, endothelial dysfunction, and the blood brain barrier are responsible for the development of CAR T-cell associated NT.
Neurotoxicity associated with CAR-T vary from person to person and the headache, pain, memory loss, dizziness, alterations in mental status, such as somnolence, disorientation, impaired attention, etc. are the most common symptoms of CAR T-cell associated NT. In addition to this, movement disorders, impaired speech, seizures, and encephalopathy to coma are also associated with CAR-T related neurotoxicities.
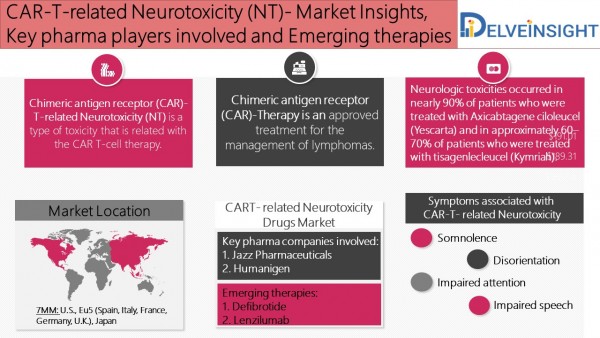
The key fact from the report:
“Neurotoxicity associated with CAR-T vary from person to person and the headache, pain, memory loss, dizziness, alterations in mental status, such as somnolence, disorientation, impaired attention, etc. are the most common symptoms of CAR T-cell associated Neurotoxicity.”
Key benefits of the report:
1. The report covers the descriptive overview of CART-related Neurotoxicity (NT) , explaining its causes, signs and symptoms, pathophysiology, diagnosis and currently available therapies
2. Comprehensive insight has been provided into the CART-related Neurotoxicity (NT) epidemiology and treatment in the 7MM
3. Additionally, an all-inclusive account of both the current and emerging therapies for CART-related Neurotoxicity (NT) are provided, along with the assessment of new therapies, which will have an impact on the current treatment landscape
4. A detailed review of CART-related Neurotoxicity (NT) market; historical and forecasted is included in the report, covering drug outreach in the 7MM
5. The report provides an edge while developing business strategies, by understanding trends shaping and driving the global CART-related Neurotoxicity (NT) market
Request for sample pages: https://www.delveinsight.com/sample-request/cart-related-neurotoxicity-nt-market
The patients who were treated with CAR-T-cells therapy, the most commonly occurring neurological symptoms were encephalopathy and headache.
Key pharma companies involved:
1. Jazz Pharmaceuticals
2. Humanigen
Emerging therapies:
1. Defibrotide
2. Lenzilumab
Table of contents:
1. Key Insights
2. Executive Summary of CART-related Neurotoxicity (NT)
3. Competitive Intelligence Analysis for CART-related Neurotoxicity (NT)
4. CART-related Neurotoxicity (NT): Market Overview at a Glance
5. CART-related Neurotoxicity (NT): Disease Background and Overview
6. Patient Journey
7. CART-related Neurotoxicity (NT) Epidemiology and Patient Population
8. Treatment Algorithm, Current Treatment, and Medical Practices
9. Unmet Needs
10. Key Endpoints of CART-related Neurotoxicity (NT) Treatment
11. Marketed Products
12. Emerging Therapies
13. CART-related Neurotoxicity (NT): Seven Major Market Analysis
14. Attribute analysis
15. 7MM: Market Outlook
16. Access and Reimbursement Overview of CART-related Neurotoxicity (NT)
17. KOL Views
18. Market Drivers
19. Market Barriers
20. Appendix
21. DelveInsight Capabilities
22. Disclaimer
23. About DelveInsight
Download a full report on CART-related Neurotoxicity (NT) – Market Insights, Epidemiology, and Market Forecast-2030
Related reports:
CART-related Neurotoxicity (NT) – Epidemiology Forecast to 2030
CART related Neurotoxicity (NT) Pipeline Insight, 2020
Media Contact
Company Name: DelveInsight Business Research LLP
Contact Person: Kritika Rehani
Email: Send Email
Phone: 9193216187
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: Nevada
Country: United States
Website: https://www.delveinsight.com/